Tune-401 Clinical Study: Information for Participants
How is CHB (chronic HepB) treated?
There are several existing treatments already available for CHB – including targeted antivirals (such as TDF/Viread®) and antibodies that can limit the activity of the virus and slow the progress of liver disease.
But none of these treatments stops the disease outright. Once you stop taking pills or injections, the virus reactivates, and disease and liver damage restart.
What are the risks and benefits?
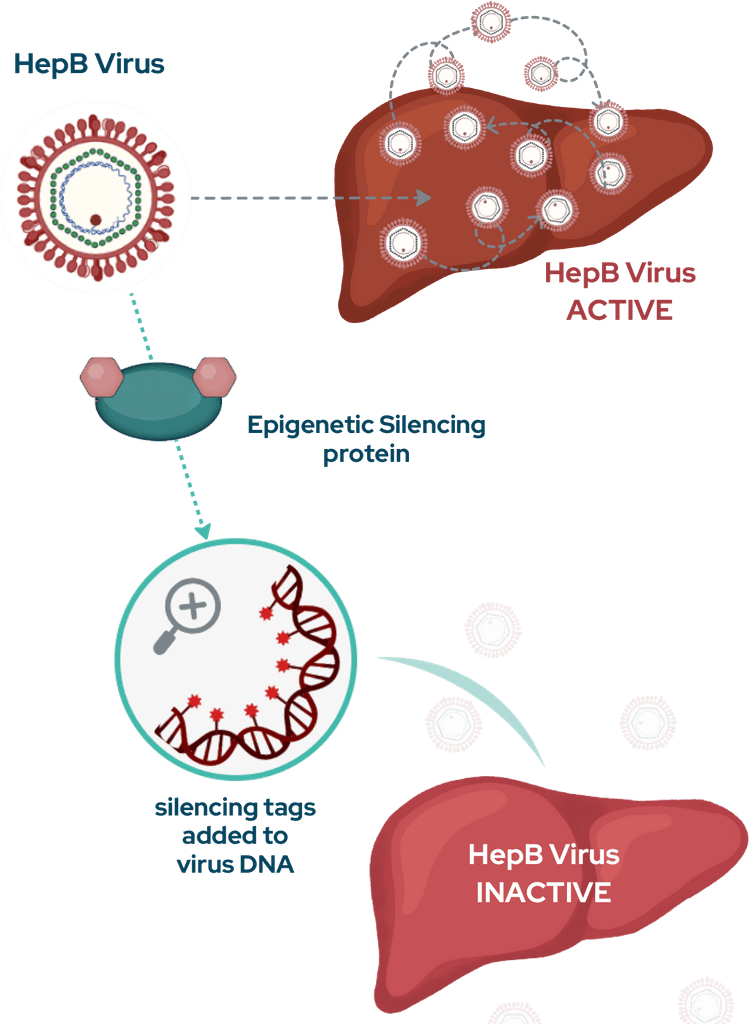
Tune-401 is an investigational drug that has not yet been approved for the treatment of chronic HepB. Since no clinical data are currently available, its precise effects and side effects are not yet known.
Tune-401 has the theoretical potential to achieve lasting control of HepB infection following a single, one-time treatment. But for some patients it may not last, or may work less well.
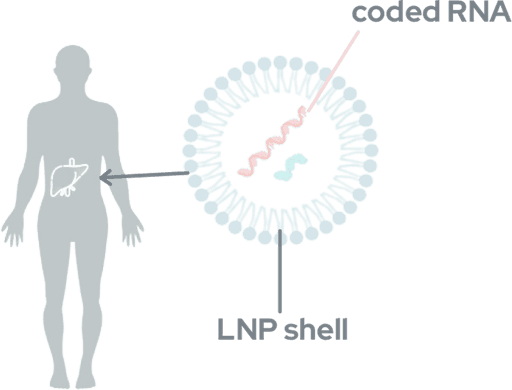
Using Lipid Nanoparticles (LNPs) to deliver drugs like Tune-401 can create liver toxicity and immune/allergic reactions in some patients. Although typically mild and short-lived, symptoms may range from headache and nausea to acute allergic reactions.
For this reason, every individual in this small-group study will be carefully monitored throughout the trial, and treated for adverse reactions and effects.